As far as I can tell battery research seems to consist of mixing every single element with lithium, and seeing if it makes a battery.
Technology
This is a most excellent place for technology news and articles.
Our Rules
- Follow the lemmy.world rules.
- Only tech related content.
- Be excellent to each another!
- Mod approved content bots can post up to 10 articles per day.
- Threads asking for personal tech support may be deleted.
- Politics threads may be removed.
- No memes allowed as posts, OK to post as comments.
- Only approved bots from the list below, to ask if your bot can be added please contact us.
- Check for duplicates before posting, duplicates may be removed
Approved Bots
That’s because lithium is in the most electropositive group of elements and sodium/potassium are too reactive for current technology. Theoretically I think Na and K based batteries should perform better as they’re even more electropositive than Li.
(Forgive the spelling error in the picture but it was the simplest one I could find quickly)
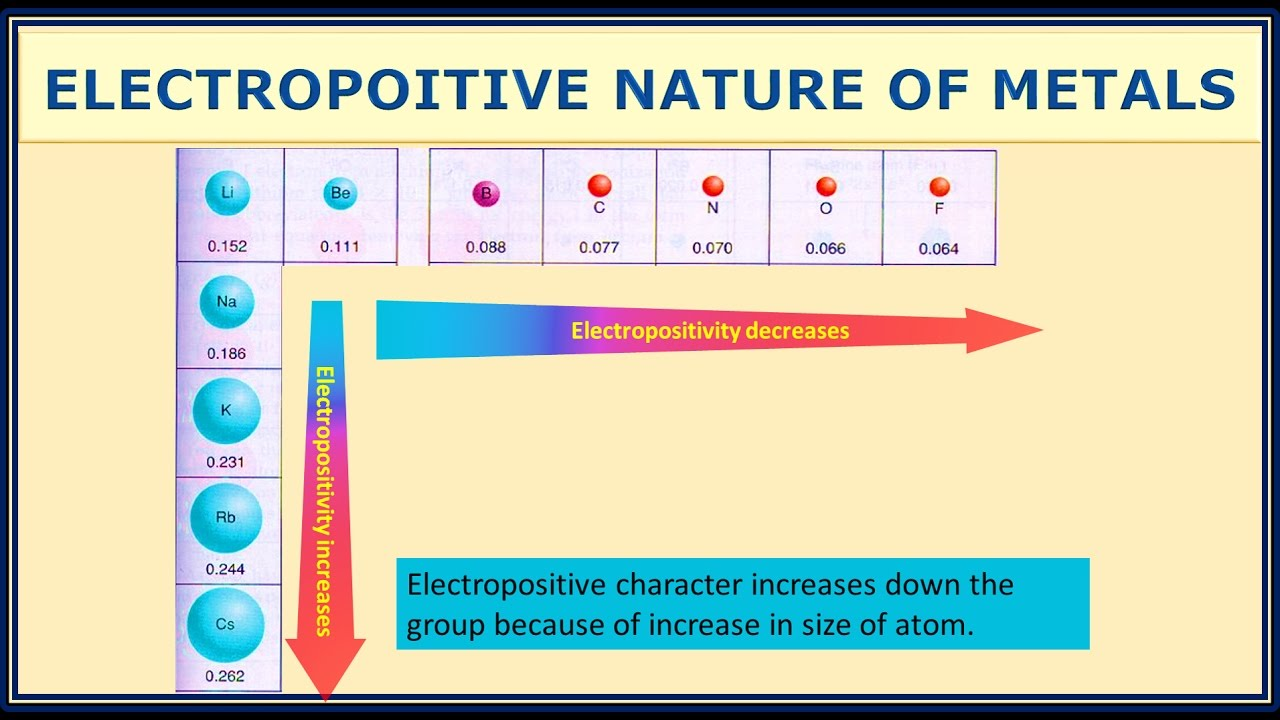
Na and K based batteries should perform better
What I'm hearing is throw some salt on a banana and power my phone for days.
I wasn't very good at chemistry.
It’s the difference in electronegativity that makes the battery. That’s why you see lithium and oxygen a lot; lithium doesn’t want electrons, oxygen does want them. Sodium and potassium are very close in electronegativity so the salty banana battery wouldn’t be good.
I’m waiting for the cesium / fluorine battery, should theoretically be awesome. Or extremely explosive
-I’m waiting for the cesium / fluorine battery, should theoretically be awesome. Or extremely explosive
I wonder how much it would cost to personally attempt this experiment.. (starts hunting for renters insurance)
The other thing for lithium is that its light, VERY light, which of course is ideal for hand sets. Manufacturers love the light and slim designs even though consumers would prefer to have a handset that can go 7 days without a charge
This is more accurate than you would think. I've seen people synthesize a new inorganic compound, and is then more or less forced by supervisors to test it as an intercalation host for Li- or Na-ion batteries without really having thought through whether that makes sense at all.
Li is small, and as long as there is room for it (sites for it to sit when intercalated and paths to diffuse through the material), and there is some species that can accommodate the additional charge (as one Li+ is introduced into the material, there needs to be a charge compensation to maintain charge neutrality - typically this is a transition metal cation that is reduced from a higher oxidation state to a lower one). In that sense a lot of materials could serve as hosts, and depending on the intercalation potential, it could be used as a cathode (LiCoO2 for instance, where the intercalation potential vs. Li/Li+ is so high that it makes for a good cathode) or an anode (LTO for instance, where the intercalation potential vs. Li/Li+ is so low that it rather makes sense to pair it with a high potential cathode, and instead make for a more niche application where things such as safety is more coveted). That said, only three structure types have been widely used commercially as intercalation hosts for Li-ion batteries: layered rocksalt types (like LiCoO2 and its deriviates, NMC and NCA), spinels (LiMn2O4 or LTO) or olivines (LiFePO4, or LFP).
Li-S is not someone randomly mixing Li with some other elements though, it has been researched for a long time and is considered one of several "holy grails"
Change lithium with Group IV elements and that's also how semiconductors are made: playing around with different impurities.
You aren't so far away from the truth!
To make a battery you need to have something that holds negative electrical charge and something with a positive electrical charge and both need to be able to change to a different state when you use it or reverse that change when you charge it.
Lithium is the lightest and smallest metal, meaning for the same size and electrical charge, your battery will weigh less.
Then you just need to find ways to make two kind of lithium compounds which have different electrical charge and can be changed between two states.
And if it doesn't explode when a child throws their battery powered bear on the ground, that would also be a good characteristic.
Flow batteries specifically don't use Lithium and are very promising in price to performance https://www.theguardian.com/australia-news/2025/jan/03/flow-batteries-are-the-future-of-renewable-energy-and-australia-could-be-a-world-leader-if-theres-funding
“Fully charged in 12 minutes” is meaningless without a capacity.
Or indeed how much it weighs.
Ok it has the 'capacity' to charge in 12 minutes - can you smell smoke?
Pretty much any battery can charge in 12 minutes if you can handle their thermal constraints.
A decent lipo can charge at 5C, but it significantly reduces the cycle life. They are claiming 1000 cycles at that charge rate.
I agree, the title is pretty useless.
Even something like "Fully charged a double A battery in 28 seconds" would've been useful/interesting.
It's not, if you charge any capacity with 1C, it will take an hour. Looks like they achieved stable charging at over 4C (charging current in amperes 4x larger than stated capacity in amp-hours).
EDIT: C is not Coulomb in this case
The point being, if it only works in the lab for minimal capacities, it's never going to see the light of production.
How do you think batteries started out?
There are tons of technologies that are inherently unscalable. Or won't be for another 50 years. Commercial unviability is one thing, but physic limitations are another matter.
True, but that doesn't mean this is one of them.
That said, I think salt batteries will eclipse these.
What are you referring to when you say "salt batteries"?
Be pretty hard to put a molten salt battery in a cellphone or electric vehicle...
A Coulomb is basically a number of electrons, so it still very much depends on capacity. The only way it could avoid capacity dependence is if the amperage varied depending on total available uncharged capacity. That in itself is unlikely because the wires that transmit the electricity can only handle so many amps before getting too hot and melting apart, so any charging system must necessarily be constructed with intended charging capacity and rate in mind from the beginning.
In this case, C refers to the current rate, not the unit Coulomb. It is a standard way of giving the current rate in battery research, and 1C is defined, as oldfart says, as the current rate required to charge that particular battery fully (to its nominal capacity) in one hour. 2C is twice this, so it is charged in half an hour, and C/2 is half this, so it is charged in two hours.
It is a convenient way of giving the current rate, because it allows a more application focused comparison (i.e. my EV battery or phone battery should be able to charge fully in one hour), but it hides the actual capacity of the battery (you have no way of knowing, without additional information, if the cell has a small or a large capacity).
ETA: The last point here is what deranger and AwesomeLowlander is getting at. You can have a very small battery with very little active material, and charge that at 10C and achieve reversible cycling for many, many cycles, and it is meaningless if it cannot be scaled to a larger cell (unless we are only considering microbatteries for example). Usually, results at a small scale is not directly transferable to larger scale, and you encounter all kinds of challenges as you scale up.
I know I have a "swords united" meme with C/c at the center but can't find it for the life of me.
What solbear said. I edited my post to clarify i did not mean the SI unit.
Indeed. A modern Nissan Leaf with a 62 kWh battery can charge in a little over 11 minutes if you have a 2kV 160 amp line to toss into it. Because you know, it's completely safe and cool to deal with those kinds of values for the average consumer.
I've read news about better battery technology for YEARS, and then nothing. Repeat the cycle.
Let me know when it's released to the public and actually usable.
In my lifetime, about the only rechargeable battery the average person had in their home was the one in their car. Now we've added 4 new major battery chemistries to the commercial space, some with multiple variants within them and all with improvements throughout their lifetimes. This is what science and technology looks like. The results you're looking for would be magic or wishful thinking.
But the battery technology did improve in these years.
You're expecting revolution. You're getting evolution.
How many years?
The amount of utility I accidentally extract from my phone over the course of a day on one charge is pretty incredible.
You need to look at battery lab research on a 10-20 year time before it gets commercialized at scale.
Moreover, go look at your rechargeable batteries from 10 or 20 years ago. They’re heavier, less energy dense, have shorter lifespans, have much slower charge rates. A lot of those advancement started in a lab and look many years to make it to your laptop or car.
The latest Chinese smartphones use new S/C batteries. They have a capacity of 6000-7000 mAh with even less overall weight.
It doesn't have to be better it just has to be Goodenough.
Goodenough died in 2023.
Yet his legacy lives on.
Well, it only looks like nothing because our power demands have increased as well.
Current Lithium Ion Polymer batteries are a far cry from the ones of a decade ago, despite being very similar tech.
The main issue with most of these alternative battery approaches are either low capacity, or low charge cycles. Finding a chemistry that both packs enough power in a small enough package to run devices for long term, and that don't wear out quickly is difficult.
Writing headlines is a selection process. You write about all the useless but cool stuff while ignoring all the boring but important stuff.
Improving Li-ion by 1% doesn’t make headlines, but that sort of stuff has been going on in the background for a few decades already. That’s why current batteries are so useful and widespread.
Lab prototypes are sexy, even if they’re 50 years away from becoming commercially viable. Sure, these things can charge fast, or hold a huge capacity, but they also tend to die after 10 cycles. Fixing that is going to take a long time, just like it did for Li-ion batteries.
Xiaomi 11i Hypercharge can go from 0% to 100% in 15 minutes. And it's a 2021 phone.
Even under rapid charging conditions with a full charge time of just 12 minutes, the battery achieved a high capacity of 705 mAh g⁻¹, which is a 1.6-fold improvement over conventional batteries. Furthermore, nitrogen doping on the carbon surface effectively suppressed lithium polysulfide migration, allowing the battery to retain 82% capacity even after 1,000 charge–discharge cycles, demonstrating excellent stability.
Assuming that this is scalable for production... Which is a big if for many of these "breakthroughs", then this could replace current Lithium Ion batteries in most devices with a noticeable bump in capacity. Everything else is pretty par for the course though with current technologies.
The full charge time is meaningless without knowing what capacity they were working with. And a quick skim didn't seem to have that in the article.
I dont give a shit fucking care untill i can buy it. Ive heard a million "this new battery tech will change the world" headlines.
New battery tech HAS changed the world though. We wouldn't have modem EVs, smartphones, etc with the batteries of yesteryear.
Then get out of technology and start reading posts in buy it for life.